Boron (B) is one of the most underrated mineral elements not only in plant but also in human nutrition. There are many reasons for this, but let’s focus here on plant and soil science.
Boron has been only recently recognised as an essential nutrient, and we keep discovering more about its roles in plant physiology.
Boron is essential because it has a structural role in the cell wall, which is a key factor in plant health. It’s heavily linked to the ability of plants to protect themselves from pests and diseases, because it regulates the production of phenols: some of these (phytoalexins) are used by plants to kill pathogens when a wound opens; others are instrumental to lignification, that is the formation of strong, fibrous or woody tissues that help the plant resist the elements and also pest attacks. For these, reasons, it is often said that Boron, Calcium and Silicon form a trio that confers plants great pest and disease resistance.
Boron also seems to control sugar and carbohydrate transport from the leaves to the rest of the plant, and therefore it’s critical for flower, fruit and seed (including grains) formation. Let’s not forget that up to 60% of the sugars fixed via photosynthesis in the leaves end up feeding soil microbes, so Boron is crucial in helping plants build soil. It is also a micronutrient for yeast, bacteria and some species of green algae, whereas it can be toxic for fungi. Azotobacter, one of the most important free-living nitrogen-fixing bacteria in the soil is very Boron-hungry!
Boron is the most important Calcium synergist, and because Calcium governs the transport of all other nutrients, this makes Boron pretty important too! Indeed, Gary Zimmer said that Calcium is the trucker of all minerals, and Boron is the steering wheel.

Where is Boron found and how does it get into a plant?
Boron is usually present in soil water as boric acid, which is also the form absorbed by plants. There is an issue with boric acid, though. In contrast with many other soluble nutrients, it is electrically neutral; this means that it cannot weakly “attach” to clay surfaces (in an exchangeable form), but it can get really “stuck” to some clays (non-exchangeable adsorption); this means that sandy soils leach Boron readily, while clay soils have most of their Boron locked and unavailable. Fortunately, boric acid is complexed and stabilised by soil humus, and this is where most of Boron is found in healthy soils.
Soils high in organic matter (as we often have in no-dig) have usually got good levels of Boron (1-2.5 ppm is optimal). This is the case, for instance, in our beds which gave a result of 2.1 ppm in a recent test. It’s important to notice that in order for organically-held Boron to become available (mineralisation), moisture is really crucial - so mulches are very useful too.
Unfortunately though, Boron is not available in a soluble form in soils that are poor of organic matter, and therefore it’s one of the most common deficient micronutrients in plants. This is not only due to its interaction with clay and leachability in sand, but also the fact that it's taken up at low pH (acidic conditions). This can be an issue in very alkaline soils, although plants partner with microbes to buffer the pH in the rhizosphere (area close to the roots) to whatever is needed for nutrient uptake or disease protection. So the more alive your soil, the less important pH is!
Some agronomists use a refractometer to help identify a Boron deficiency. If the Brix levels of a crop do not drop overnight, this is a sign of a deficiency. Boron triggers a large flush of sugars fixed during the day are released from the chloroplasts down to the roots. When Boron is insufficient, sugars get trapped in the leaves and the Brix level does not drop overnight.
Plants that require and contain high quantities of Boron are Sugar beets, Brassicas, Carrots, Soy and also green manures such as Clover and Alfalfa.
Optimal Boron nutrition is known to reduce the incidence of Clubroot in Brassicas, Wart in Potatoes, Tobacco mosaic virus in Beans, Fusarium wilt, Yellow leaf curl virus and Powdery mildew in Tomatoes.
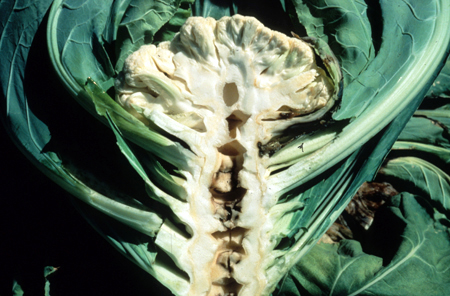
Because Boron is immobile in the plant, deficiency generally affects plant growing points, such as buds, fruits, flowers, and root tips. Plants low in boron may produce deformed flowers, aborted seeds, thickened, brittle, puckered leaves, or dead growing points. Examples you may have come across are “stem crack” in Celery and “hollow stem disorder” in Broccoli or Cauliflower.
It has been shown that in all of these cases, a small dose of Boron applied at the correct time could make the difference in terms of crop quality. This is very important not only for marketability of vegetables, but also for human nutrition, because Boron deficiency causes the reduction of two of the most important anti-oxidants: vitamin C and glutathione.
Ok, so, how do we address Boron deficiency? Well, first of all: don’t use synthetic NPK fertilisers, as they have been shown to inhibit the uptake of Boron from the soil.
Secondly, another easy answer: compost! In no-dig or any organic method, Boron should not be an issue, if compost or manures are applied correctly.
Commercial growers usually apply Boron both as soil drench and foliar sprays, and its efficiency is amplified when this is done in conjunction with humic acid which stabilises its effect. It has been shown that foliar sprays are much less effective if Boron is deficient in the soil, so it makes sense to address the soil first. The cheapest and most common way to supplement Boron is by using Borax, which is a naturally-occurring salt version of boric acid.
By the way, Borax can also be used for human consumption (at very low dosage), and you would be shocked to know how effective research says it is for brain, bone, hormonal and metabolism performance. Boron supplementation has been shown to speed up bone fracture healing dramatically (I will put a reference in the comments). As usual, don’t take any medical advice from me, and let’s go back to plants.
Although Borax is extremely cheap, as with many other garden inputs, it is a resource that has to be harvested (from seasonal lakes in mountainous or desert areas) and transported over long distances. So using compost is a much better option than Borax!
Another thing to bear in mind is that Borax is toxic if applied in excess - it is a known fungicide and herbicide, as well as a poison used for pest control. But this is only true if applied in overly large dosage. And the optimal range depends on the quantity of Calcium present in the soil.
This is because of the interaction between Boron and Calcium; as previously said, Boron improves Calcium efficacy in plants, but Calcium in the soil increases the demand for Boron.
I’ll end on a culinary note: as beer experts know, Boron helps to convert maltose, and there is some evidence that a similar process might take place in soya and derivatives (tofu, soy milk, etc.) So I wouldn’t be surprised if the flavour profile of our crops was also related (among many other things) to the Boron availability of our soil.
References
Update on human health effects of boron Journal of Trace Elements in Medicine and Biology, 28, 4, 2014. [link]
Article by Walter Last on the use of Borax in humans, and how, historically, the pharmaceutical industry has been actively lobbying to have it controlled [link]
Marschner's Mineral Nutrition of Higher Plants (2011) [link]
Mineral Nutrition and Plant Disease (Huber et al, 2007) [link]
Graeme Sait's Nutrition Farming podcast [link]
The Nature and Structure of Soils (Weil, Brady, 2016) [link]











Comments